Capillary action, a fascinating phenomenon observed in water and other fluids, describes the tendency of liquids to move up narrow spaces against the force of gravity. This remarkable property is essential for various natural and engineered systems, from the transport of water in plants to the design of irrigation systems.
At the heart of capillary action lies the interplay between cohesive forces within a liquid and adhesive forces between the liquid and the solid surfaces it contacts. Water, with its high surface tension due to hydrogen bonding, is particularly prone to capillary action. The surface tension of water, which is the force per unit length that causes the liquid’s surface to contract, plays a crucial role in this process.
In the context of water, capillary action is responsible for moving groundwater from wet regions of soil to dry areas, aiding in the distribution of moisture to plant roots. This principle is harnessed in subirrigation designs, such as wicking beds and sub-irrigated planters, to supply water to plants efficiently.
In plants, capillary action is integral to the functioning of xylem, the tissue responsible for transporting water and nutrients from the roots to the leaves. The water forms concave menisci inside the xylem’s pores, allowing it to rise against gravity.
Capillary action also plays a role in weathering processes, where it can draw water into rock fractures, contributing to the breakdown of rocks over time.
The significance of capillary action extends beyond natural systems. It is a fundamental concept in materials science, where it influences the behavior of liquids in confined spaces and is relevant to the design of microfluidic devices and other applications.
In summary, capillary action is a ubiquitous phenomenon that underpins the movement of water in various contexts, from the microscopic scale within plants to the macroscopic scale in soil and geological processes. Its importance in natural and engineered systems underscores the critical role water plays in our world.
Citations:
https://en.wikipedia.org/wiki/Capillary_action
[glossary_wikipedia]Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space in opposition to or at least without the assistance of any external forces like gravity.

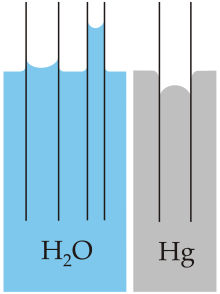
The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube such as a straw, in porous materials such as paper and plaster, in some non-porous materials such as clay and liquefied carbon fiber, or in a biological cell.
It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to propel the liquid.